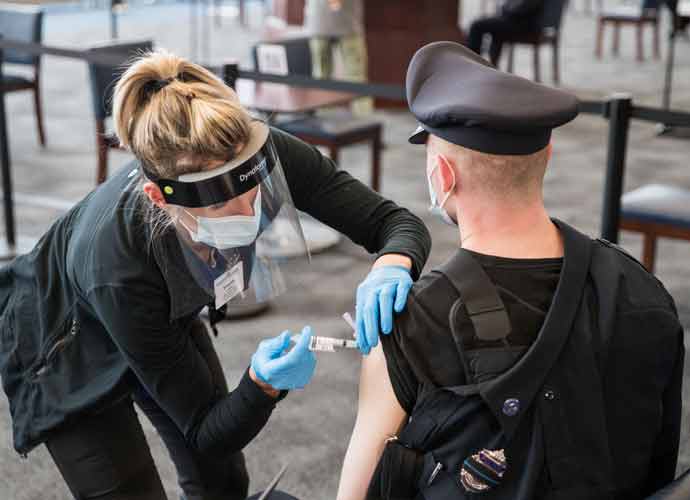
Moderna Asks For FDA Approval Of Fourth Covid-19 Vaccine Dose
Moderna joined Pfizer-BioNTech by asking the FDA for authorization of a fourth vaccine to halt the spread of COVID-19.
Phizer and BioNTech’s proposed vaccine is a second booster for seniors, ages 65 and up. Moderna’s request expands upon Phizer’s Tuesday appeal, asking for authorization for a vaccine aimed at all adults.
The request comes as an omicron variant, BA.2, is making the rounds through Europe. Moderna listed a report out of Israel that found that a second booster would decrease rates of infection as the reason for their request.
The current recommendation includes the first two doses of the vaccine and then a dose of the booster five months later, or two months after receiving the Johnson & Johnson vaccine.
Subscribe to our free weekly newsletter!
A week of political news in your in-box.
We find the news you need to know, so you don't have to.
Studies show that both Pfizer and Moderna’s vaccine protection rates decrease over a four-month span, so people who have gotten the booster are more likely to show zero to mild symptoms when contracting the virus. In February, the Centers for Disease Control and Prevention found that the vaccines were 91% effective against hospitalizations after two months, but by four months after the booster, they were only 78% effective.
The current CDC recommendations include the original two shots for children 5-11 and the vaccine and booster for everyone who is 12 and up.
Get the most-revealing celebrity conversations with the uInterview podcast!








Leave a comment