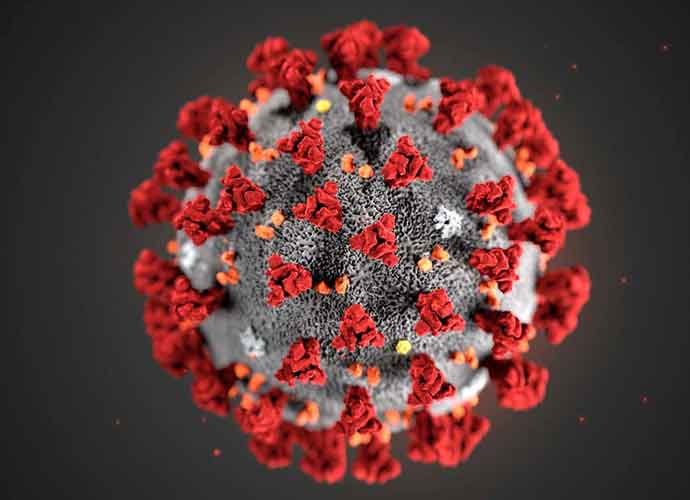
U.S. Study Shows AstraZeneca Vaccine Is Safe & Effective, But Doubts On Data Remain
AstraZeneca announced on Monday that a late-stage clinical trial of the Oxford/AstraZeneca coronavirus vaccine found the shot effectively prevented COVID-19 and reported no evidence of the thrombosis-related events that have concerned E.U. countries.
According to the U.S.-based Phase 3 study, the vaccine was able to prevent all severe disease and hospitalization and 79 percent of symptomatic cases of COVID-19. The vaccine was 80 percent effective among participants 65 years and older.
Data collected from the 32,449 participants showed “no safety concerns related to the vaccine,” said AstraZeneca.
But just hours after the results were announced, U.S. scientists said Astra/Zeneca may have not shared all of its data with regulators.
Subscribe to our free weekly newsletter!
A week of political news in your in-box.
We find the news you need to know, so you don't have to.
The trial’s independent data safety monitoring board specifically analyzed the thrombosis results and found no increased risk. There were no rare cerebral venous sinus thrombosis instances, which was a worry of Germany’s Paul-Ehrlich-Institut.
The drug regulator gave the Oxford/AstraZeneca vaccine the all-clear last week after its safety committee PRAC concluded the vaccine is “not associated with an increase in the overall risk of thromboembolic events or blood clots.”
After these results, most countries that had stopped distributing the vaccine resumed administration. However, Denmark reported one death and one hospitalization after the shot over the weekend, so their operations remain suspended.
Get the most-revealing celebrity conversations with the uInterview podcast!






Leave a comment