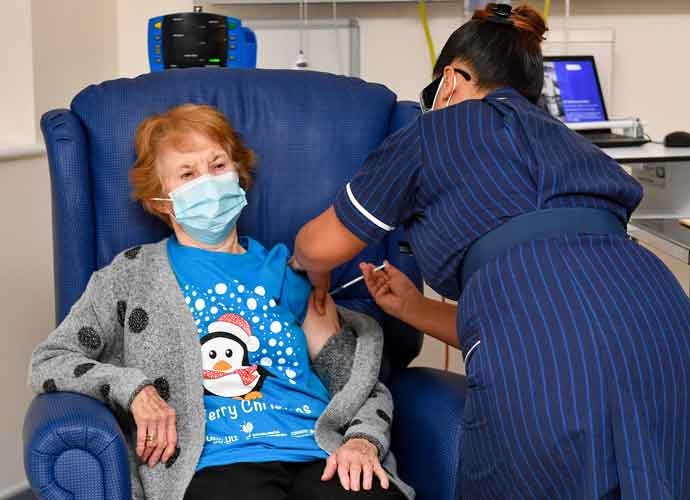
COVENTRY, ENGLAND - DECEMBER 08: Margaret Keenan, 90, is the first patient in the United Kingdom to receive the Pfizer/BioNtech covid-19 vaccine at University Hospital, Coventry, administered by nurse May Parsons, at the start of the largest ever immunisation programme in the UK's history on December 8, 2020 in Coventry, United Kingdom. More than 50 hospitals across England were designated as covid-19 vaccine hubs, the first stage of what will be a lengthy vaccination campaign. NHS staff, over-80s, and care home residents will be among the first to receive the Pfizer/BioNTech vaccine, which recently received emergency approval from the country's health authorities. (Photo: Getty)
Early Tuesday morning, the Food and Drug Administration (FDA) and the Center for Disease Control and Prevention (CDC) issued concurring orders to halt distribution of the Johnson & Johnson COVID-19 vaccine. The administrations based their decision off reports that six women contracted blood clots soon after vaccination.
“All six cases occurred among women between the ages of 18 and 48, and symptoms occurred 6 to 13 days after vaccination,” the FDA and CDC said in their joint statement. “In these cases, a type of blood clot called cerebral venous sinus thrombosis (CVST) was seen in combination with low levels of blood platelets (thrombocytopenia). We are recommending a pause in the use of this vaccine out of an abundance of caution.”
Of the women who contracted blood clots, one has since died and another is in critical condition. Johnson & Johnson later issued their own statement complying with FDA and CDC recommendations.
“We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine,” reads a new official Johnson & Johnson statement. “In addition, we have been reviewing these cases with European health authorities. We have made the decision to proactively delay the rollout of our vaccine in Europe.”
Subscribe to our free weekly newsletter!
A week of political news in your in-box.
We find the news you need to know, so you don't have to.
White House COVID-19 coordinator Jeff Zients confirmed that the Johnson & Johnson halt will not change the United States’ COVID-19 vaccine rollout schedule. “[The halt] will not have a significant impact on our vaccination plan: Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date,” Zients confirmed in an interview Tuesday. “The United States has secured enough Pfizer and Moderna doses for 300 million Americans.”
Florida Gov. Ron DeSantis said Floridians have the right to drive into crowds of protestors…
Former President Barack Obama issued a warning about the rapid descent toward autocracy. The former…
Earlier this month, Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. announced that…
President Donald Trump’s plans for his “big, beautiful bill” are raising concerns from many conservative…
The Trump Administration is cracking down on immigrants living in the U.S. illegally by enforcing…
National Intelligence Director Tulsi Gabbard is exploring new ways to present President Donald Trump's intelligence…