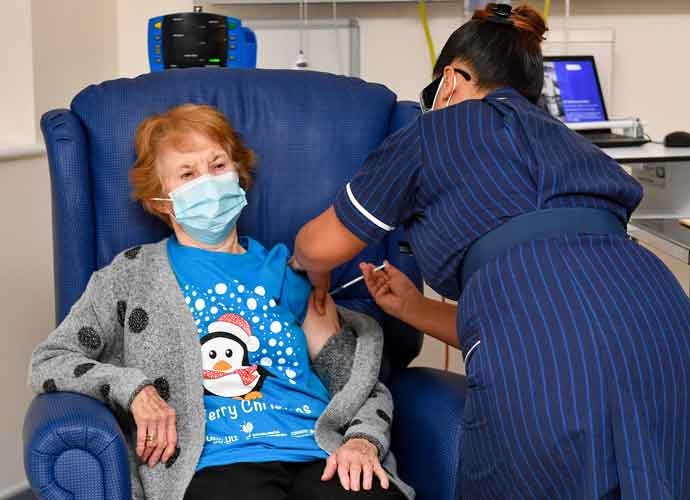
COVENTRY, ENGLAND - DECEMBER 08: Margaret Keenan, 90, is the first patient in the United Kingdom to receive the Pfizer/BioNtech covid-19 vaccine at University Hospital, Coventry, administered by nurse May Parsons, at the start of the largest ever immunisation programme in the UK's history on December 8, 2020 in Coventry, United Kingdom. More than 50 hospitals across England were designated as covid-19 vaccine hubs, the first stage of what will be a lengthy vaccination campaign. NHS staff, over-80s, and care home residents will be among the first to receive the Pfizer/BioNTech vaccine, which recently received emergency approval from the country's health authorities. (Photo: Getty)
Pharmaceutical company Johnson & Johnson’s single-dose COVID-19 vaccine has proven to be safe and considerably effective in preventing severe symptoms of the virus.
The Food and Drug Administration (FDA) released a briefing analyzing results from a Johnson & Johnson clinical trial in preparation for an independent advisory panel scheduled for Thursday. The panel will discuss if Johnson & Johnson’s vaccine should be authorized for children under 18 years old.
The FDA’s findings confirmed that the Johnson & Johnson vaccine was 66% effective in all trials against COVID-19. During the 28-day clinical trial, none of the subjects were hospitalized for COVID-19 related symptoms. Johnson & Johnson’s vaccine, however, proved 42.3% effective in people over 60 with preexisting health risks like obesity, high blood pressure, or other heart problems which is below the FDA’s requirement of 50% minimum effectiveness for authorization. The administration is expected to perform more tests; the clinical trial hosted notably fewer people with preexisting conditions.
Similar to two-dose vaccines, members of the Johnson & Johnson clinical trial reported very mild fatigue, pain at site of injection and headaches in the days after the vaccine was administered. One subject required surgery for blood clots, which appeared days after the vaccine’s injection. Johnson & Johnson has since commented that the clotting is not related to the vaccine, instead saying the rate clots occurred in the trial matches the rate of the general population. In their brief, the FDA wrote it “will recommend surveillance for further evaluation of thrombolytic events with deployment of the vaccine into larger populations.”
Subscribe to our free weekly newsletter!
A week of political news in your in-box.
We find the news you need to know, so you don't have to.
Thursday’s panel will also likely decide the timeline for roll out of Johnson & Johnson’s vaccine.
Former U.S. President Joe Biden continues to defend his decision to drop out of the…
Two years after Sen. John Fetterman (D-Pennsylvania) checked himself into a hospital to treat his…
https://youtu.be/-5rxJ1A4uHU Pro-Palestine protesters faced off with officers during a rally in a Columbia University library…
A resurfaced audio recording reveals Matt Moran, the top political strategist for Virginia Gov. Glenn…
The U.S. Supreme Court allowed President Donald Trump's administration to place a ban on transgender…
On Monday, Israel’s cabinet approved a plan to reoccupy the Gaza Strip for an indefinite…